Each step of drawing the lewis structure of O 3 is explained in detail in this tutorial. Find the Total Number of Valence Electrons.

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
In the lewis structure of NH 3 you can see there are one double bond and one single.

. This type of Lewis dot structure is represented by an atomic symbol and a series of dots. Here are the steps that I use to draw a Lewis structure. What are the steps in drawing a Lewis structure.
First determine the central atom. Add electrons for negatively charged ions. The video covers the basic Lewis structures youll see in an introductory chemistry class.
A video tutorial for how to draw Lewis Structures in five steps. Decide which atom is the central atom in the structure. How to Draw a Lewis Structure.
H2O Lewis structure NH 3 Lewis structure Step 01. Write the correct skeletal structure for the molecule. Group number for each element of valence electrons.
Draw a trial structure by putting electron pairs around every atom until each gets an octet. Attach the terminal atoms using pairs of electrons. They only want to form one bond to get to a noble gas configuration.
Draw the simple structure skeleton structure of the compound by connecting everything with single bonds only. For all but the simplest molecules the following step-by-step process is faster. A single bond results when ___ electrons are shared between atoms.
Erin Orazem Created Date. List the steps for drawing a Lewis structure for an ionic compound. Write the skeleton structure of the molecule.
Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Add up the total number of valence electrons found in the entire compound. Determine the total number of valence electrons.
Following are the steps to construct the Lewis Structure. In this step add up the total number of valence electrons from all the atoms in the molecule. If there are multiple elements that have more dots than the rest then just pick one at random among the higher dotted elements.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Lewis Dot Structures Practice Sheet page 3 Steps for Drawing Lewis Dot Structures for Larger Molecules 1. Calculate the valence electrons.
The trial-and-error method for writing Lewis structures can be time consuming. The SUM TOTAL is what is important. Fill the octet of the terminal atoms.
Place the remaining electrons on the central atoms. Find the Number of Electrons Needed to Make the Atoms Happy. Drawing amine formulas from names.
A polyatomic anion or cation add. Each step of drawing lewis structure of. Count the valence electrons in your trial structure.
Subtract electrons for positively charged ions. Hoeger 1 Determine the total number of valence electrons for ALL atoms. Lewis structure of water molecule contains two single bonds around oxygen atom.
LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. A Draw the lewis structure by repeating steps 1-4. Put remaining valence electrons as lone pairs.
Turn lone pairs into bonds as each atom fulfill electron octet rule. Add electrons to all the noncentral atoms. In short these are the steps you need to follow for drawing a Lewis structure.
A Hydrogens H and halogens FClBrI are almost always outer atoms. Sum the valence electrons from all the atoms. Calculation of total valence electrons of NH3 Step 02.
See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. COUNT THE TOTAL VALENCE ELECTRONS. Find the Total Number of Valence Electrons.
Dont be concerned with which atom gave what. Valence electrons are the electrons present in the outermost shell of the electronic configuration of an atom. Draw the Lewis Dot Structure for the Hydrogen atom.
The rules allow for several types of draws. O 3 lewis structure. Drawing Lewis Structures A step-by-step Guide by C.
Steps of drawing lewis structure of BH 3. Steps for drawing a Lewis structure of compounds. O Water - Drawing Steps.
Use two valence electrons to form each bond in the skeleton structure. The video covers the basic Lewis structures youll see in an introductory chemistry class. Since Hydrogen is in Group I it has one 1 valence electron in its shell.
All valence electrons of the atoms in Lewis structures must be shown. Draw the Lewis structure for CaCl. Count the valence electrons of atoms To draw Lewis structure we need to figure out the number of valence electrons in individual atoms as shown in the table below.
Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps. NH3 Lewis structure N 2 Lewis structure Step 01. Hydrogen atoms are always terminal only one bond Put more electronegative elements in terminal positions.
If the entire molecule is charged ie. Count the total number of valence electrons. After drawing the lewis structure of NH 3 you can decide shape of the O 3 molecule.
In Lewis dot structure only valence electrons are used for making of the structure. Generally electrons are paired. ACDChemSketch is an easy-to-use chemically intelligent molecular structure drawing application with more than 2 million users worldwide.
Connect four atoms via single bonds. Determine the central atom. Pg 17 text Author.
Unpaired electrons are observed in odd electron molecules such as NO and NO2. Refer to the first picture b find the elements that has more dots than the rest. Determine the Number of Bonds in the.
Follow these simple steps to correctly draw a Lewis dot structure. Central atom N. Steps for drawing a Lewis Structurepdf - STEPS FOR DRAWING A LEWIS STRUCTURE.
Instructions for Drawing Lewis Structures For molecules and polyatomic ions composed of nonmetals. Refer to the second picture. Such bonds can be represented by _____.
A double bond results when ___ electrons are shared between atoms. Draw the Lewis structure for KCl.

How To Draw Lewis Structures A Step By Step Tutorial Middle School Science Blog

Lewis Structures 5 Steps 1 Count Valence E Available If An Anion Add Charge To Valence E If A Cation Subtract Charge From Valence E 2 Draw Skeleton Ppt Download

Chemistry 101 Draw Lewis Dot Structures For Covalent Compounds Youtube

Lewis Diagrams Made Easy How To Draw Lewis Dot Structures Youtube
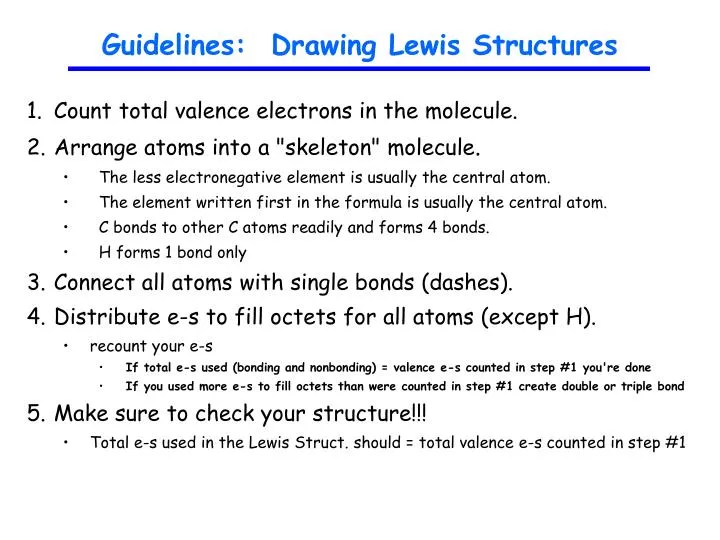
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Youtube

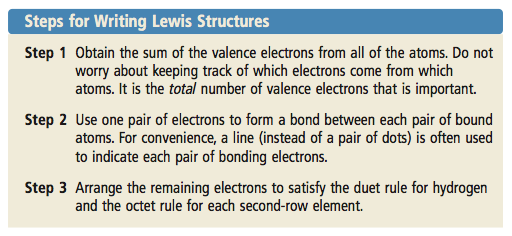
0 comments
Post a Comment